Frequently asked questions and answers for IMPROVAC®.
IMPROVAC is a safe and reliable vaccine that uses the pig's own immune system against boar taint.
Boar taint is an unpleasant odour or taste that many consumers would notice if they cooked or ate pork from male pigs that had reached puberty. It has been compared to the smell of urine, faeces and sweat. Boar taint most often occurs in pork from male pigs that were not castrated. Most male pigs today are castrated, preventing the presence of boar taint in pork, although there are a few countries (UK, Ireland, Australia and others) where the practice is not routine and instead male pigs are normally slaughtered at a young age. To learn more about boar taint, please refer to the questions towards the end of this FAQ or visit www.boartaint.com
IMPROVAC stimulates the pig’s immune system to produce antibodies that ultimately block and reverse the accumulation of compounds responsible for boar taint.
No. IMPROVAC has no direct effect in eliminating boar taint. it works only via the immune system. The testes of sexually mature boars produce steroids such as androstenone which cause boar taint. Testicular activity also reduces the ability of the liver to eliminate a substance called skatole, which is produced by bacteria in the gut and which also contributes to taint. IMPROVAC inhibits testicular activity, preventing the production of androstenone and also allowing the liver to more effectively metabolise and eliminate skatole. It takes about 2 weeks for all the androstenone and skatole to be eliminated after the second dose of IMPROVAC is administered.
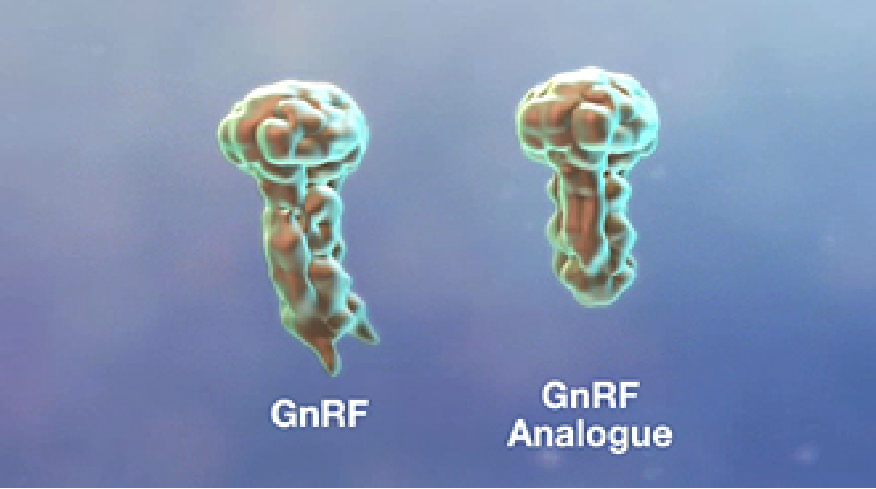
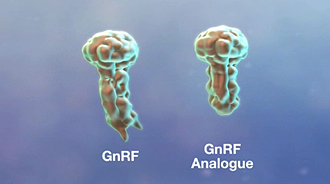
The antigen in IMPROVAC is a synthetic incomplete analogue of gonadotropin-releasing factor (GnRF) linked to a carrier protein to make it immunogenic, that is, to help trigger the immunological response. The combination is similar enough to natural GnRF to help trigger antibodies that neutralise natural GnRF, BUT different enough NOT to have hormonal activity. The carrier protein is one that is used widely in many human vaccines.
Synthetic GnRF analogue in IMPROVAC is similar to but different from natural GnRF.
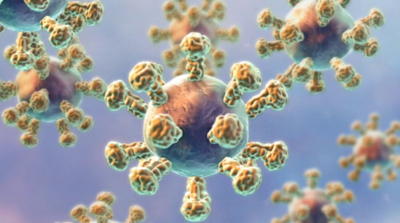
Synthetic GnRF bound to the surface of a large immunogenic carrier protein.
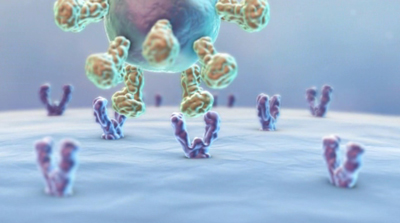
The IMPROVAC antigen cannot bind to the pituitary receptor and thus has no hormonal activity.
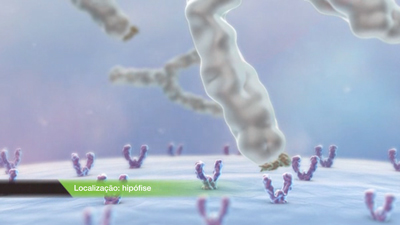
The antibodies induced by the IMPROVAC antigen recognise the middle and larger end of natural GnRF and neutralise it, thus preventing natural GnRF from binding to the pituitary.
No. IMPROVAC is not a hormone; it is a vaccine. IMPROVAC has no direct hormonal or drug activity.
No. IMPROVAC does not contain any genetically modified components; it is made without the use of genetic engineering.
Yes. Regulatory authorities around the world have granted IMPROVAC a 0-day withdrawal time, indicating that pork could safely be consumed from pigs at any time after injection, although in practice the period will be at least 4 weeks. Human food safety is assured because, like most vaccines currently used in pig production, IMPROVAC’s active substance is a protein and is designed to work only when injected. It has been shown in many high-dose studies to lack any activity or effect when administered orally. Pork from pigs given IMPROVAC has been consumed safely in countries such as Australia and New Zealand since 1998.
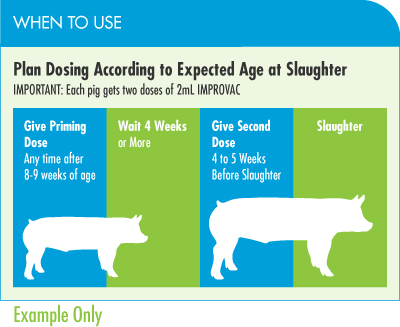
The first dose has no effect on testicular function or on boar taint. The first dose of IMPROVAC simply “primes” the pig’s immune system so that it is ready to respond quickly to the second dose and eliminate the compounds that cause boar taint.
Field experience suggests that the risk of self-injection is very small. If it occurs, however, accidental self-injection may produce similar effects in people to those seen in pigs. These may include a temporary reduction in sexual hormones and reproductive functions in both men and women and an adverse effect on pregnancy. The risk of these effects occurring is greater after a second or subsequent accidental injection than after a first injection. A single injection, however, may prime the immune system to react to subsequent injections, in the same way it does in the pig so anyone experiencing a single injection should not use the product again.
If a person receives an accidental injection of IMPROVAC, they should wash the injury thoroughly with clean running water and seek prompt medical advice, taking the package leaflet with them. Because a single injection may prime the immune system to react to subsequent injections in the same way as it does in the pig, the person should avoid injecting IMPROVAC in the future.
Zoetis has been actively involved in the development of injection devices with enhanced safety features that greatly reduce the risk of self-administration. Such devices must be used for administering IMPROVAC and all users should receive thorough training on correct vaccination procedures before starting to use IMPROVAC.
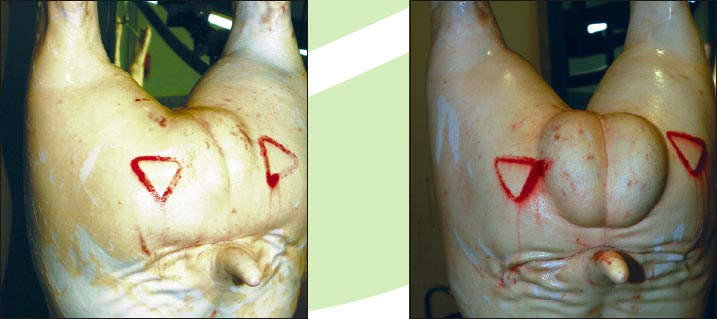
Yes. Compared to physical castrates, the most obvious difference is that there are testicles present. These are much smaller than in normal, non-immunized boars because their development has been blocked. However, the visibly larger testicles in non-immunized boars compared with IMPROVAC boars will enable slaughterhouses to tell straightaway if a pig might not have been correctly given IMPROVAC.
No. Meat from pigs vaccinated with IMPROVAC looks, tastes and smells the same as pork that is currently produced from physically castrated or female pigs. Repeated consumer studies have confirmed that it has no discernible trace of boar taint and the pork is of the same high eating quality as meat currently produced from castrated or female pigs.
For the producer, the main benefit of IMPROVAC is a more cost-effective and profitable way of producing high-quality pig meat free of boar taint without recourse to physical castration or to slaughter at a light live weight.
For the processor, the main benefit of IMPROVAC is a higher-yielding, leaner carcass with the same high eating quality and freedom from boar taint. It provides the ability to market high-quality pork produced in a more animal-friendly and environmentally sustainable manner.
For the consumer, the main benefit of IMPROVAC is the production of safe, high-quality pork in an animal-friendly and environmentally sustainable manner.
For the pig, the main benefit of IMPROVAC is the elimination of the pain and stress of physical castration.
The short answer is yes. If used according to label directions, IMPROVAC will control boar taint in pigs slaughtered at an older age. However, the vast majority of pigs are slaughtered before weeks 26–27 in most markets. The two-dose IMPROVAC regimen, where the second dose is given around 19–21 weeks, effectively eliminates boar taint and results in smaller testes at slaughter.
When pigs are slaughtered at an older age than 27 weeks and the second dose is delayed until approximately 22–23 weeks, problems may arise. In this situation, IMPROVAC will control boar taint, but because the pigs are more mature at the time of the second dose, they will have larger testes, and thus producers and processors may be confused as to whether the pigs have been correctly dosed.
In these older boars, producers may also observe sexual and aggressive behaviours becoming a problem before the second dose is administered. In this situation, producers may need to discuss a tailored immunization program with their veterinarian.
IMPROVAC temporarily suppresses testicular function, including testosterone production, and the behaviour of fully vaccinated animals is similar to that of physically castrated animals. In most pig production systems, male behaviours are unlikely to be a problem before the normal time of the second injection (22 weeks of age or less) and possibly beyond this, depending on the breed of pig and the management system. Behaviour problems are minimized when pigs are maintained in stable social groups with no mixing with unfamiliar animals. Producers producing heavy pigs and observing problems of sexual and aggressive behaviour in their animals may need to consider a tailored immunization program and should consult their veterinarian.
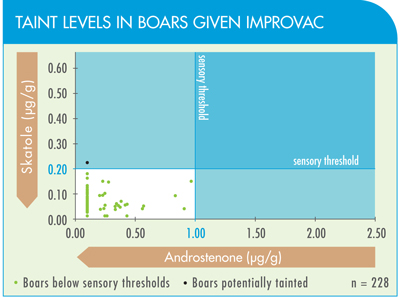
No management system can guarantee that all meat will be free from taint. Even females and castrated pigs can occasionally be affected by taint.
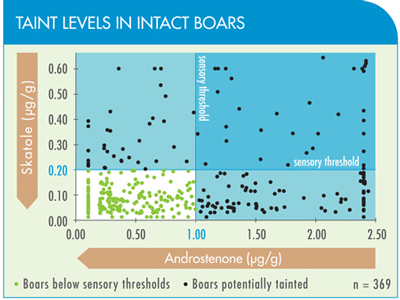
It is important to realize that the current management system, which produces females and physical castrates, is not 100% effective in eliminating taint as detected by either chemical analysis or, more importantly, olfactory analysis. For example, a recent U.S. survey revealed that 1–3% of gilts and barrows had high androstenone and/or skatole concentrations that were reflected in high taint scores on olfactory analysis.
This background level of taint may be a result of a low number of cryptorchids or missed castrates (on which IMPROVAC will be effective) and/or as a result of skatole (a key taint substance) being absorbed via the skin from a wet and soiled environment.
However, an evaluation of over 2,000 boars in a commercial setting showed that less than 1% of boars vaccinated with IMPROVAC carried a high risk of taint. IMPROVAC achieved an effectiveness rate in controlling boar taint comparable to that achieved with physical castration.
The bottom line is in the eating experience, and extensive consumer testing has confirmed that even highly sensitive individuals can detect no difference between meat from IMPROVAC boars and physically castrated or female pigs.
There is no age restriction and IMPROVAC may be used in boars of any age. It should not be used in boars intended for breeding, but it may be used in mature boars no longer required for breeding purposes. The normal directions for use should be followed.
Consumers benefit from IMPROVAC because they get the same high-quality pork as from physically castrated pigs, but produced in a more animal-friendly and environmentally sustainable manner.
Because it provides a more efficient and animal friendly alternative to the physical castration of piglets. It means that male pigs can be raised as boars for most of their lives and thus make more efficient use of feed and produce a quality carcass, but without any risk of tainted meat or boar-like behaviour. IMPROVAC ensures more sustainable and environmentally friendly food production by reducing the amount of feed required and waste produced compared to castration based systems. In other words IMPROVAC adoption is important because it enables humane and sustainable production of high eating quality pork.
Non-castrating markets:
Because it provides consumers with consistently high quality pork – so they don’t waste money on meat they do not enjoy as much as they could. Pork produced using IMPROVAC is free of boar taint and has been proven at least as succulent and juicy as other pork on the market. The only other way to guarantee pork with such a consistent, good flavour is physical castration; this practice is bad for the animal’s welfare and bad for the environment because castrates produce more waste than non-castrated boars. In addition IMPROVAC gives the flexibility to raise male pigs to a much heavier weight without typical boar-like behaviour such as aggression, which can also be a welfare problem when male pigs are kept together.
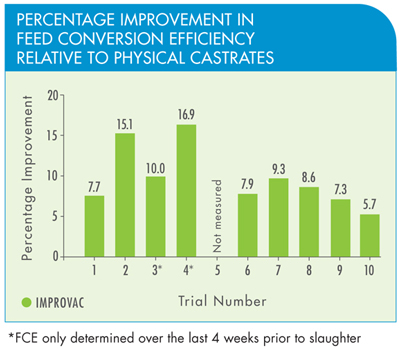
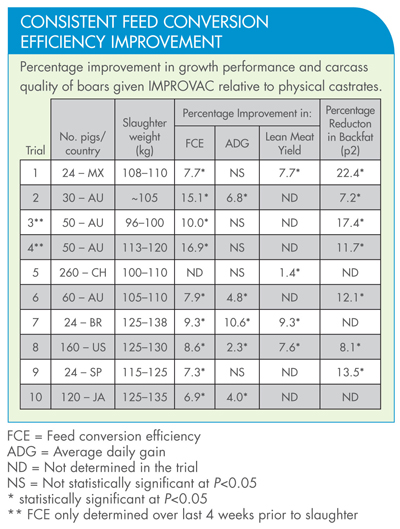
Studies have shown that pigs given IMPROVAC are more efficient at converting food into body weight compared to physically castrated pigs. This means that they use less agricultural resources per kilo of meat produced and generate less waste. On a global scale, assuming an annual production of 600 million boars, this would save 36 million tonnes of food and reduce waste products by 11 million tonnes (assuming 10% less faeces from pigs given IMPROVAC).
IMPROVAC was originally developed in Australia as part of a collaboration between a Government Department of Agriculture research station and a local animal health company. It was launched in 1998 in Australia where it has been very successful. However, CSL did not have a global presence in the animal health market. When CSL and its products were acquired by Zoetis in 2004 the company realised the potential for IMPROVAC to revolutionise swine production across the globe and started to develop the product accordingly.
IMPROVAC is being progressively introduced around the world and is currently registered in over 60 countries, including US, Canada, Japan, China, Australia, New Zealand, Switzerland, Russia, Brazil, Mexico, Chile, Thailand, Korea, South Africa, the Philippines and the entire European Union.